
Discussing latest trends end emerging threats in the field of cyber security, vulnerability management, and cyber threat intelligence.
Dive into scientific discoveries and technological advancements
Created by users on Jellypod • Updated daily

Discussing latest trends end emerging threats in the field of cyber security, vulnerability management, and cyber threat intelligence.

An audio version of the best of apiaristsadvocate.com's monthly content.

Hosted by Andie Cartwright, Marketing Manager of Life Sciences at PointClickCare. Andie delves into the critical changes within aging and vulnerable populations and how data can change the quality of life for those individuals who are often overlooked and under served.
Kernziel für die Landwirte, Lohnunternehmer und AMAZONE ist es, dass wir ein hohes Ertragsniveau pro ha Fläche mit nachhaltigen Methoden schaffen. Es gilt daher, die Effizienz der Produktionsprozesse insbesondere durch Innovation und Digitalisierung zu verbessern und so präzise wie möglich die Pflanzen zu behandeln. Dieser Podcast wurde per KI zusammengestellt. Die ursprünglichen Inhalte stammen von Menschen – sie wurden erarbeitet, strukturiert und zusammengetragen.

<p> <strong>Suggested LBT experiments & metrics (what to run and measure)</strong></p><ul><li><p><strong>Baseline</strong>: run codex without cymatic/particles, then enable particles only, then cymatics only, then both. Compare results.<br /><br /></p></li><li><p><strong>Metrics</strong>:<br /><br /></p><ul><li><p>Particle count vs time (creation/destruction).<br /><br /></p></li><li><p>Cluster size distribution (assembly sizes).<br /><br /></p></li><li><p>Phase coherence metric in patches: R=∣⟨eiθ⟩∣R = |\langle e^{i\theta}\rangle|R=∣⟨eiθ⟩∣ per patch (Kuramoto order param).<br /><br /></p></li><li><p>Exchange events count (inter-universe transfers).<br /><br /></p></li><li><p>Local entropy vs branch events correlation.<br /><br /></p></li></ul></li><li><p><strong>Parameter sweeps</strong>:<br /><br /></p><ul><li><p>Sweep lambda_C (cymatic coupling) to see phase-bias effect on particle motion and clustering.<br /><br /></p></li><li><p>Sweep P_adhere and A_thresh for assembly regimes.<br /><br /></p></li><li><p>Sweep exchange_base to explore inter-universe transfer regimes.<br /><br /></p></li></ul></li><li><p><strong>Visualizations</strong>:<br /><br /></p><ul><li><p>Animated E/V maps with particle overlays (colored by phase).<br /><br /></p></li><li><p>Phase-field maps of cymatic φ and order parameter R.<br /><br /></p></li><li><p>Time-series of particle cluster histograms.<br /><br /></p></li></ul></li></ul><p></p>

SatYield Live is our weekly podcast where we break down global crop yield trends using satellite data and AI. Each episode dives into the latest insights, regional highlights, and key signals from the field—helping traders, analysts, and agri-professionals stay ahead of the curve.

<p>Come si trasforma l'apprendimento in un'esperienza davvero coinvolgente? In questo podcast esploriamo il confine sottile tra gioco e didattica. Attraverso il dialogo tra una docente esperta e due studenti dalle visioni opposte (Luca e Maria) facciamo chiarezza sui pilastri del <em>Learning Experience Design</em>. Dalla distinzione cruciale tra Gamification e Serious Games, all'analisi dei bisogni psicologici di Maslow applicati al gaming, fino alla personalizzazione per i diversi stili cognitivi. Un viaggio teorico e pratico per chi vuole capire come la meccanica del gioco possa potenziare la mente.</p>

Coming to a city near you. Stay up to date with the new breeders and unique special events.

Overview of Paddy's work in the field of genetic genealogy

Un podcast a tre episodi che esplora come e perché i giochi ci coinvolgono, con un focus speciale sui serious games. Attraverso un dialogo leggero tra docente e studenti (tra ironia, entusiasmo e riflessione), parleremo di Magic Circle e sospensione della realtà, di flow e immersione secondo Csikszentmihalyi, e degli ingredienti chiave che rendono un gioco davvero efficace: feedback, storia, simulazione, intrattenimento e apprendimento. Un viaggio breve ma chiaro per capire come il gioco possa diventare uno spazio sicuro, motivante e formativo.

An educational podcast that goes in depth in IB subject enviornmental social science to help teach and give out tips to ace all exams

Discussing Dr Kevin M Decker's Intersectional Consciousness Theory, with a mix of hosts: Dr Kevin Decker, James, Mark , Dr Abebe Botha, Dr van der Berg, and others.

Zusammenfassungen ausgewählter Sitzungen der Vorlesung Fortgeschrittene Methoden der Statistik

The Honors Element is a podcast created for Penn State Honors General Chemistry students, exploring the fundamental ideas that shape how we understand the chemical world. Each episode connects core concepts to real-life applications while preparing students for upcoming lectures.

Una docente esperta, Veronica Rossano, e due studenti universitari discutono il mondo dei Serious Games, approfondendo il concetto di "Magic Circle" e la sospensione della realtà. La serie presenta casi studio in ambito sanitario e sociale, evidenziando l'impatto educativo di questi giochi. In chiusura, si analizzano le dinamiche creative del brainstorming per trasformare idee in progetti concreti.

Host: Stavo tornando a casa da scuola, come ogni pomeriggio, quando il mio smartwatch ha vibrato in modo strano. Host: All’inizio ho pensato fosse una notifica di un amico, ma sullo schermo è comparsa una scritta: "Messaggio urgente dal futuro". Nova: Identità: Nova. Intelligenza artificiale dell’anno 2035. Il tuo futuro è in pericolo. Host: Mi sono fermato, incredulo. Non sapevo se stavo sognando o se fosse reale. Nova: Il collasso ecologico è vicino. Per evitarlo, ho bisogno del tuo aiuto. Ti invierò tre indizi. Seguili. Host: Lo schermo ha mostrato l’immagine di un fiume quasi asciutto. All’inizio non l’ho riconosciuto, poi ho capito: era quello vicino al parco dove andavo da bambino. Nova: Vai a vederlo. Host: Non sapevo cosa aspettarmi, ma qualcosa dentro di me si è mosso. Curiosità? Paura? Non lo so. Host: Ho deciso di andare al parco. Sapevo che quello era solo l’inizio della mia missione.
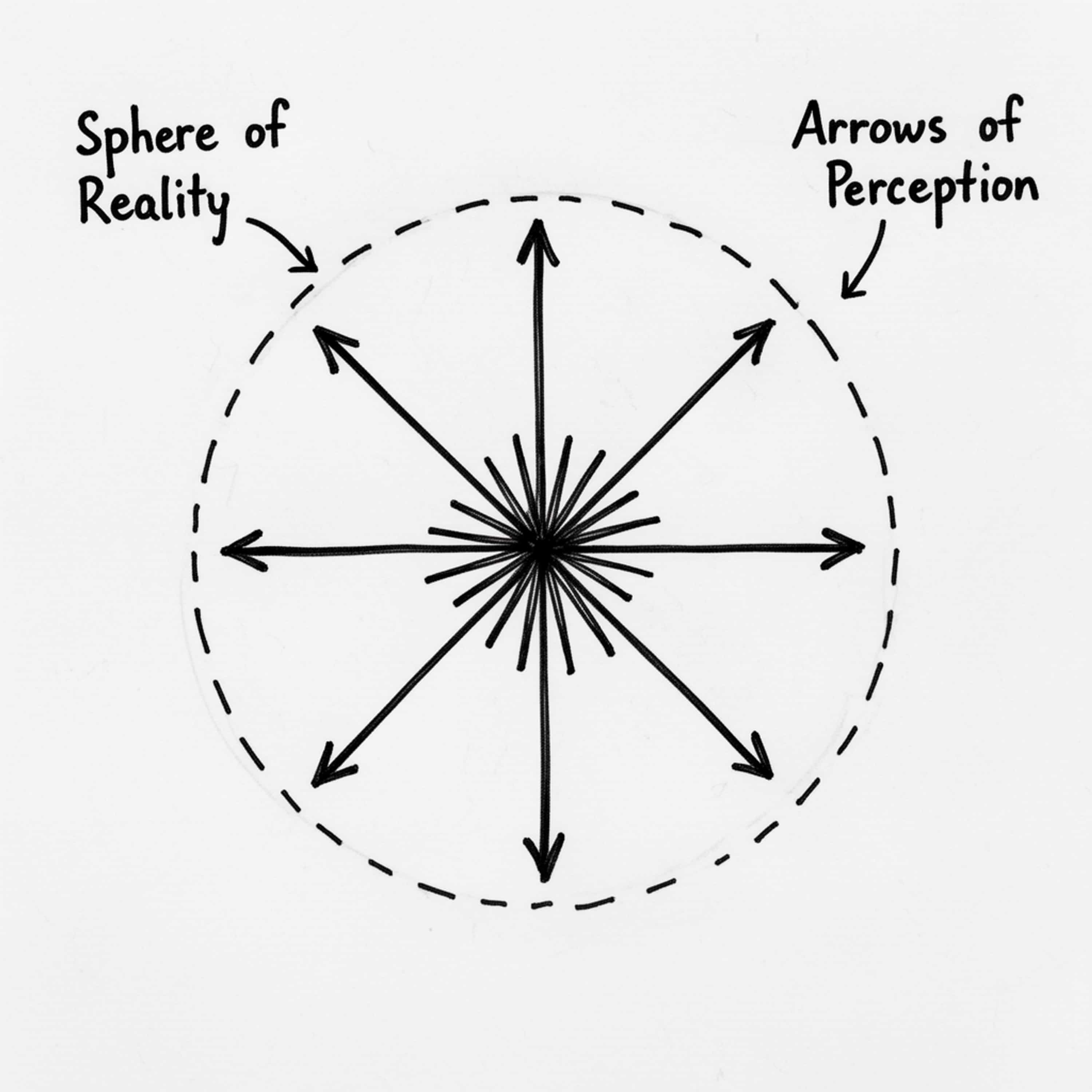
Twenty-five years ago, I drew a simple sketch: a point at the center, arrows radiating outward, a dashed boundary around it all. I didn't fully understand what I was drawing. I just knew it was true. It took me twenty-five years to find the words. This podcast introduces the Toye Paradox — a logical proof that consciousness cannot be falsified — and builds from there to a complete framework for understanding how we expand or contract our reality. The core insight is simple: you are always locked at the center of your own experience. You cannot perceive from anywhere else. You cannot step outside to verify what others see. There is no "outside the box." But the boundary of your reality is not fixed. Through awareness and action, it expands. Through withdrawal and fear, it contracts. The size of your world is determined not by your potential, but by what you actually do. Over ten episodes, we explore: Why consciousness cannot be explained from within itself What lies beyond the boundary of your current reality How action — not intention — moves the boundary Why bigger spheres encounter more (it's geometry) What actually happens when two realities make contact The three possible outcomes of relationship The chain from action to expansion to contact to connection This is not self-help. This is not motivation. This is a framework — rigorous, practical, and honest about what it can and cannot explain. I'm Toye Oyelese. I'm a family physician, a father, and someone who has spent decades thinking about how human beings navigate uncertainty. This is The Sphere of Reality.

Company of Cooks partners with Dynamic Earth to merge culinary arts with science, revealing the intriguing science behind everyday food. This series uncovers innovative cooking techniques and the fascinating chemistry that enhances flavor and nutrition. Discover how science transforms your meals into extraordinary experiences.

Join us to learn more about the Pittsburgh Reptile Show & Sale the largest monthly reptile expo in Western Pennsylvania

hoe de aarde eruit ziet van binnen en van buiten

Welcome to "Mind Matters," your essential podcast for mastering psychology! Join us as we explore core psychological concepts, influential theories, and groundbreaking experiments that illuminate the complexities of the human mind and behavior. Designed specifically for students, each episode breaks down key topics in an engaging and easy-to-understand way, helping you grasp complex ideas, prepare for exams, and deepen your understanding of the fascinating world of psychology. Tune in to turn intricate theories into clear insights and make your study sessions more effective!

Welcome to Educators Empowering Educators, where we bring together educator innovators and the science leaders who will partner with them in the E3 program. During these episodes, you’ll hear from the chief scientists who will join your teacher-teams, learn about their research focus in global agriculture and sustainable development, and discover how their work will integrate into the classroom. Our aim is simple: to empower educators with the latest science, and to invite scientists into the world of teaching because together, we’re building a more globally literate agriculture future.

Asia's first community supported Astronomy Podcast

A show to dispel myths around food and nutrition, inspired by age old traditional knowledge and backed by scientific research.

The break down of proofs
Want to discover more? Explore all podcasts on Jellypod